

Ion chromatography of soluble salts
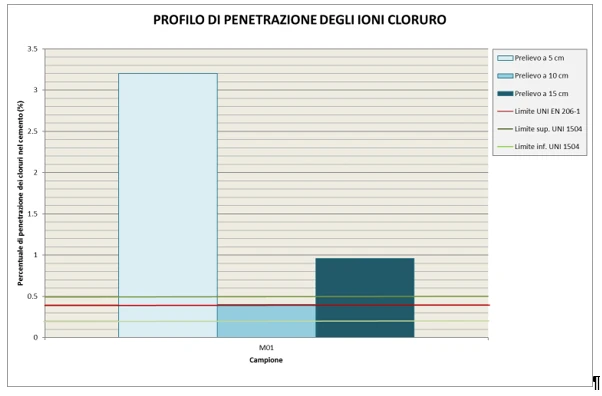
The determination of the penetration profile of chloride ions is carried out using an alternative method to UNI 9944:1992, i.e. ion chromatography, which allows the simultaneous determination of anionic species. This technique is based on the separation of analytes using an anion exchange column based on their affinity for the stationary phase. The eluent, containing the separated analytes, is then passed through a post-column chemical derivatisation device, called a suppressor, which, by exchanging protons with the mobile phase, aims to reduce the background conductivity of the eluent by forming the weak acid conjugated, and to enhance the analyte signal, which is detected using an in-line conductivity meter. The analytes are identified by comparing the retention time of the sample peaks with the retention time of reference solutions. Concentration is determined by comparing the area of the peak with the calibration curve of the analyte, which is constructed using a series of reference solutions at different concentrations. Such analyses return a percentage value of the presence of chlorides with reference to the assumed weight of concrete.
Reference standards: UNI EN 206:2021; UNI 11104:2016; UNI EN 1504.
